Furosemide
Furosemide is a drug, diuretic.
Dosage form
Granules for preparation of oral suspension (for children), solution for intravenous and intramuscular administration, tablets.
Pharmachologic effect
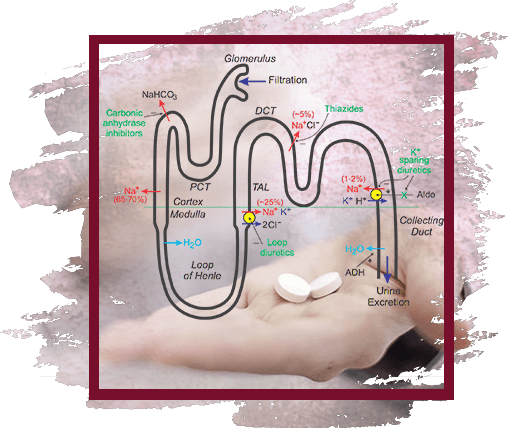
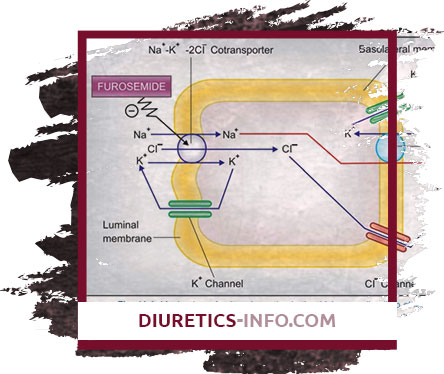
"Loop" diuretic; causes a fast, strong and short-term diuresis. It has natriuretic and chloruretic effects, increases the excretion of K +, Ca2 +, Mg2 +. Penetrating into the lumen of the renal tubule in the thick segment of the ascending knee of the loop of Henle, it blocks the reabsorption of Na + and Cl−. Due to the increase in Na + excretion, there is a secondary (mediated by osmotically bound water) enhanced excretion of water and an increase in K + secretion in the distal renal tubule. At the same time, the excretion of Ca2 + and Mg2 + increases. Has secondary effects due to the release of intrarenal mediators and the redistribution of intrarenal blood flow. Against the background of the course of treatment, there is no weakening of the effect. In heart failure, it quickly leads to a decrease in the preload on the heart through the expansion of large veins. It has a hypotensive effect due to an increase in the excretion of NaCl and a decrease in the reaction of vascular smooth muscles to vasoconstrictor effects and as a result of a decrease in the BCC. The action of furosemide after intravenous administration occurs within 5-10 minutes; after ingestion - after 30-60 minutes, maximum action - after 1-2 hours, duration of effect - 2-3 hours (with reduced renal function - up to 8 hours). During the period of action, the excretion of Na + increases significantly, but after its termination, the rate of excretion decreases below the initial level ("rebound" or "cancellation" syndrome). The phenomenon is caused by a sharp activation of the renin-angiotensin and other antinatriuretic neurohumoral regulation links in response to massive diuresis; stimulates the arginine-vasopressin and sympathetic systems. Reduces the level of atrial natriuretic factor in plasma, causes vasoconstriction. Due to the phenomenon of "rebound" when taken 1 time per day may not have a significant effect on the daily excretion of Na + and blood pressure.
Pharmacokinetics
Absorption - high, TCmax for oral administration - 1-2 hours, for intravenous administration - 30 minutes. The remaining pharmacokinetic parameters for intravenous and oral administration are the same. Bioavailability is 60-70%. The relative volume of distribution is 0.2 l / kg. Communication with plasma proteins - 98%. Penetrates through the placental barrier, excreted in breast milk. It is metabolized in the liver to form 4-chloro-5-sulfamoylanthranilic acid. It is secreted into the lumen of the renal tubules through the anion transport system existing in the proximal nephron. T½ = 0.5-1 h. It is excreted mainly (88%) by the kidneys unchanged and in the form of metabolites; with feces - 12%. Clearance - 1.5-3 ml / (min · kg).
Indications
Edema syndrome in CHF II-III degree, liver cirrhosis, kidney disease (including against the background of nephrotic syndrome); acute heart failure (pulmonary edema), cerebral edema, hypertensive crisis (alone or in combination with other antihypertensive drugs), arterial hypertension (severe), hypercalcemia; carrying out forced diuresis in case of poisoning with chemical compounds excreted by the kidneys in an unchanged form; eclampsia.
Contraindications
Hypersensitivity, acute renal failure with anuria (glomerular filtration rate less than 3-5 ml / min), severe hepatic failure, hepatic coma and precoma, stenosis of the urethra, acute glomerulonephritis, urinary tract obstruction with stones, precomatous states, hyperglycemic coma, hyperuricemia , decompensated mitral or aortic stenosis, GOKMP, increased central venous pressure (over 10 mm Hg), arterial hypotension, acute myocardial infarction, pancreatitis, impaired water and electrolyte metabolism (hypovolemia, hyponatremia, hypokalemia, hypochloremia, hypocalcemia), hypomagnesemia , digitalis intoxication.
With care. Prostatic hyperplasia, SLE, hypoproteinemia (risk of developing ototoxicity), diabetes mellitus (decreased glucose tolerance), stenosing atherosclerosis of the cerebral arteries, pregnancy (especially the first half, possibly use for health reasons), lactation.
Dosage regimen
Intravenous (rarely intramuscularly), parenteral administration is advisable in cases where it is not possible to take it orally - in urgent situations or with a pronounced edema syndrome. Edema syndrome: initial dose - 40 mg. Intravenous administration is carried out for 1-2 minutes; in the absence of a diuretic response, it is administered every 2 hours in a dose increased by 50% until an adequate diuresis is achieved. The average daily dose for intravenous administration in children is 0.5-1.5 mg / kg, the maximum is 6 mg / kg. Patients with reduced glomerular filtration and low diuretic response are prescribed in larger doses - 1-1.5 g. The maximum single dose is 2 g. Inside, in the morning, before meals, the average single initial dose is 20-80 mg; in the absence of a diuretic response, the dose is increased by 20-40 mg every 6-8 hours until an adequate diuretic response is obtained. A single dose, if necessary, can be increased to 600 mg or more (required with a decrease in glomerular filtration and hypoproteinemia). With arterial hypertension, 20-40 mg is prescribed; in the absence of a sufficient decrease in blood pressure, other antihypertensive drugs must be added to the treatment. When adding furosemide to already prescribed antihypertensive drugs, their dose should be reduced by 2 times. The initial single dose in children is 2 mg / kg, the maximum is 6 mg / kg.
Side effects
- On the part of the cardiovascular system
Decrease in blood pressure, orthostatic hypotension, collapse, tachycardia, arrhythmias, decrease in the volume of circulating blood. - From the nervous system
Dizziness, headache, myasthenia gravis, cramps of the calf muscles (tetany), paresthesia, apathy, weakness, weakness, lethargy, drowsiness, confusion. - From the senses
Visual and hearing impairment. - From the digestive system
Decreased appetite, anorexia, dry mouth, thirst, nausea, vomiting, constipation or diarrhea, cholestatic jaundice, pancreatitis (exacerbation). - From the genitourinary system
Oliguria, acute urinary retention (in patients with prostatic hypertrophy), interstitial nephritis, hematuria, decreased potency. - Allergic reactions
Purpura, urticaria, exfoliative dermatitis, exudative erythema multiforme, vasculitis, necrotizing angiitis, pruritus, chills, fever, photosensitivity, anaphylactic shock. - On the part of the hematopoietic organs
Leukopenia, thrombocytopenia, agranulocytosis, aplastic anemia. - On the part of water-electrolyte metabolism
Hypovolemia, dehydration (risk of thrombosis and thromboembolism), hypokalemia, hyponatremia, hypochloremia, hypocalcemia, hypomagnesemia, metabolic alkalosis. - Laboratory indicators
Hyperglycemia, hypercholesterolemia, hyperuricemia, glucosuria, hypercalciuria. When administered intravenously (additionally) - thrombophlebitis, renal calcification in premature babies.

Overdose
Symptoms: decreased blood pressure, collapse, shock, hypovolemia, dehydration, hemoconcentration, arrhythmias (including AV blockade, ventricular fibrillation), acute renal failure with anuria, thrombosis, thromboembolism, drowsiness, confusion, flaccid paralysis, apathy. Treatment: correction of water-salt balance and CBS, replenishment of the BCC, symptomatic treatment. There is no specific antidote.
special instructions
Against the background of the course of treatment, it is necessary to periodically monitor blood pressure, the concentration of electrolytes in plasma (including Na +, Ca2 +, K +, Mg2 +), CBS, residual nitrogen, creatinine, uric acid, liver function and carry out, if necessary, appropriate correction of treatment (with greater frequency in patients with frequent vomiting and against the background of parenterally administered fluids). Patients with hypersensitivity to sulfonamides and sulfonylurea derivatives may have cross-sensitivity to furosemide. In patients receiving high doses of furosemide, in order to avoid the development of hyponatremia and metabolic alkalosis, it is inappropriate to limit the consumption of table salt. For the prevention of hypokalemia, the simultaneous administration of K + preparations and potassium-sparing diuretics (primarily spironolactone) is recommended, as well as a diet rich in K +. An increased risk of developing disorders of water and electrolyte balance is observed in patients with renal failure. The selection of the dosage regimen for patients with ascites against the background of liver cirrhosis should be carried out in stationary conditions (disturbances in the water-electrolyte balance may lead to the development of hepatic coma). For this category of patients, regular monitoring of plasma electrolytes is indicated. If azotemia and oliguria appear or increase in patients with severe progressive kidney disease, it is recommended to suspend treatment. It is excreted in milk in women during lactation, and therefore it is advisable to stop feeding. Patients with diabetes mellitus or with reduced glucose tolerance require periodic monitoring of the concentration of glucose in the blood and urine. In unconscious patients with prostatic hypertrophy, narrowing of the ureters or hydronephrosis, urinary control is necessary due to the possibility of acute urinary retention. During the period of treatment, one should avoid engaging in potentially hazardous activities that require increased attention and speed of psychomotor reactions.
Interaction
Increases the concentration and risk of developing nephro- and ototoxic effects of cephalosporins, aminoglycosides, chloramphenicol, ethacrynic acid, cisplatin, amphotericin B (due to competitive renal excretion). Increases the effectiveness of diazoxide and theophylline, reduces - hypoglycemic drugs, allopurinol. Reduces the renal clearance of Li + preparations and increases the likelihood of developing intoxication. Strengthens the hypotensive effect of antihypertensive drugs, neuromuscular blockade caused by depolarizing muscle relaxants (suxamethonium) and weakens the effect of non-depolarizing muscle relaxants (tubocurarine). Pressor amines and furosemide are mutually ineffective. Drugs that block tubular secretion increase the serum furosemide concentration. With the simultaneous use of GCS, amphotericin B, the risk of hypokalemia increases, with cardiac glycosides, the risk of digitalis intoxication increases due to hypokalemia (for high and low polarity cardiac glycosides) and T1 / 2 lengthening (for low polarity). NSAIDs, sucralfate reduce the diuretic effect due to inhibition of Pg synthesis, changes in plasma renin concentration and aldosterone release. Reception of salicylates in large doses during therapy with furosemide increases the risk of their toxicity (due to competitive renal excretion). Intravenously administered furosemide has a slightly alkaline reaction, so it should not be mixed with drugs with a pH of less than 5.5.
Doping
Although furosemide itself is not a doping, it is used in sports medicine for the elimination of prohibited substances and, as a consequence, it itself is equated to doping drugs and banned for use in athletes by the World Anti-Doping Agency (WADA) [1]. In addition, in some non-strength sports, such as rhythmic gymnastics or synchronized swimming, where the weight of the athlete is of principle, furosemide is used as a means of losing weight, which is also considered doping.
 DE
DE FR
FR IT
IT ES
ES